Understanding the IFRA Standards
The IFRA Standards consist of a comprehensive set of guidelines, providing essential safety criteria for fragrance materials used in consumer products such as perfumes, cosmetics, and household goods. These Standards are continuously updated as scientific knowledge and understanding of fragrance safety evolve. The amendments have aimed to restrict, prohibit, or apply limits of certain ingredients that may pose potential health risks or cause adverse reactions in consumers.
Impact and challenges of the IFRA 51st Amendment on the fragrance industry
The 51st Amendment to the IFRA Standards will have several effects on the fragrance industry, impacting various aspects of its operations and practices. Let us explore the key ways in which this amendment will affect the industry:
- Formulation and product development: Fragrance houses and manufacturers will need to reformulate their existing products and create new ones to comply with the stricter guidelines outlined in the 51st Amendment. This may involve replacing or reducing the use of certain ingredients to meet the new safety standards. Additionally, companies may need to invest in research and development to find alternative ingredients that achieve similar scent profiles without compromising safety.
- Consumer trust and risk reduction: By restricting the use of specific ingredients in fragrances, the 51st Amendment aims to reduce the risk to consumers. Fragrance manufacturers will need to conduct rigorous testing and adhere to the latest scientific data on fragrance ingredients, ensuring that their products meet the newly defined safety thresholds.
- Regulatory compliance: The IFRA Standards apply to all members globally. By adhering to the IFRA Standards, fragrance manufacturers demonstrate their commitment to producing safe and reliable products, building trust among consumers.
- Research and collaboration: The 51st Amendment highlights the importance of ongoing research and collaboration within the industry. Fragrance houses will invest in scientific studies to better understand the safety and environmental impact of their products. Collaboration with experts, scientists and regulatory authorities will become essential for staying updated with the latest advancements and complying with emerging safety standards.
- Innovation and product differentiation: Complying with the 51st Amendment may lead to a wave of innovation within the fragrance industry. Manufacturers will seek creative solutions to formulate captivating scents while adhering to safety guidelines. This could lead to the emergence of unique and differentiated fragrances that appeal to environmentally conscious consumers.
Key provisions of the IFRA 51st Amendment
The 51st Amendment includes the following key changes:
- The updated guidance document for the use of the IFRA Standards.
- The IFRA Standards, as summarised below.
The Annex on contributions from other sources to the IFRA Standards, which combines – where applicable – information from natural contributions (former Annex I) and Schiff bases (former Annex II).
The IFRA 51st Amendment introduces 48 new fragrance ingredient Standards, including:
- 32 Restriction Standards based on skin sensitization and systemic toxicity.
- 11 Restriction Standards based on dermal sensitization using QRA2.
- 2 Restriction Standards based on systemic toxicity (TTC).
- 1 Restriction Standard based on depigmenting potential.
- 1 Restriction Specification Standard based on dermal sensitization and systemic toxicity.
- 1 Prohibition Standard due to potential genotoxicity effects.
Additionally, 12 existing standards have been revised. There are minor updates to 2 existing Standards, where additional CAS numbers have been listed in the Revised Restriction Standard for Farnesol and the Revised Prohibition Standard for Mintlactone. Notwithstanding the updates, they are not affecting the content of the Standards on Farnesol and Mintlactone. Thus, the implementation timelines associated with the 51st Amendment do not apply for the Standards on Farnesol and Mintlactone. The extra CAS Numbers for these two substances should be considered immediately.
IFRA 51st Amendment implementation timelines
Notification of the 51st Amendment to the IFRA Standards was published on the 30th of June 2023. The implementation timeline for NEW and EXISTING creations is summarised in the image below (applies to the new Restriction and Restriction Specification Standards only).
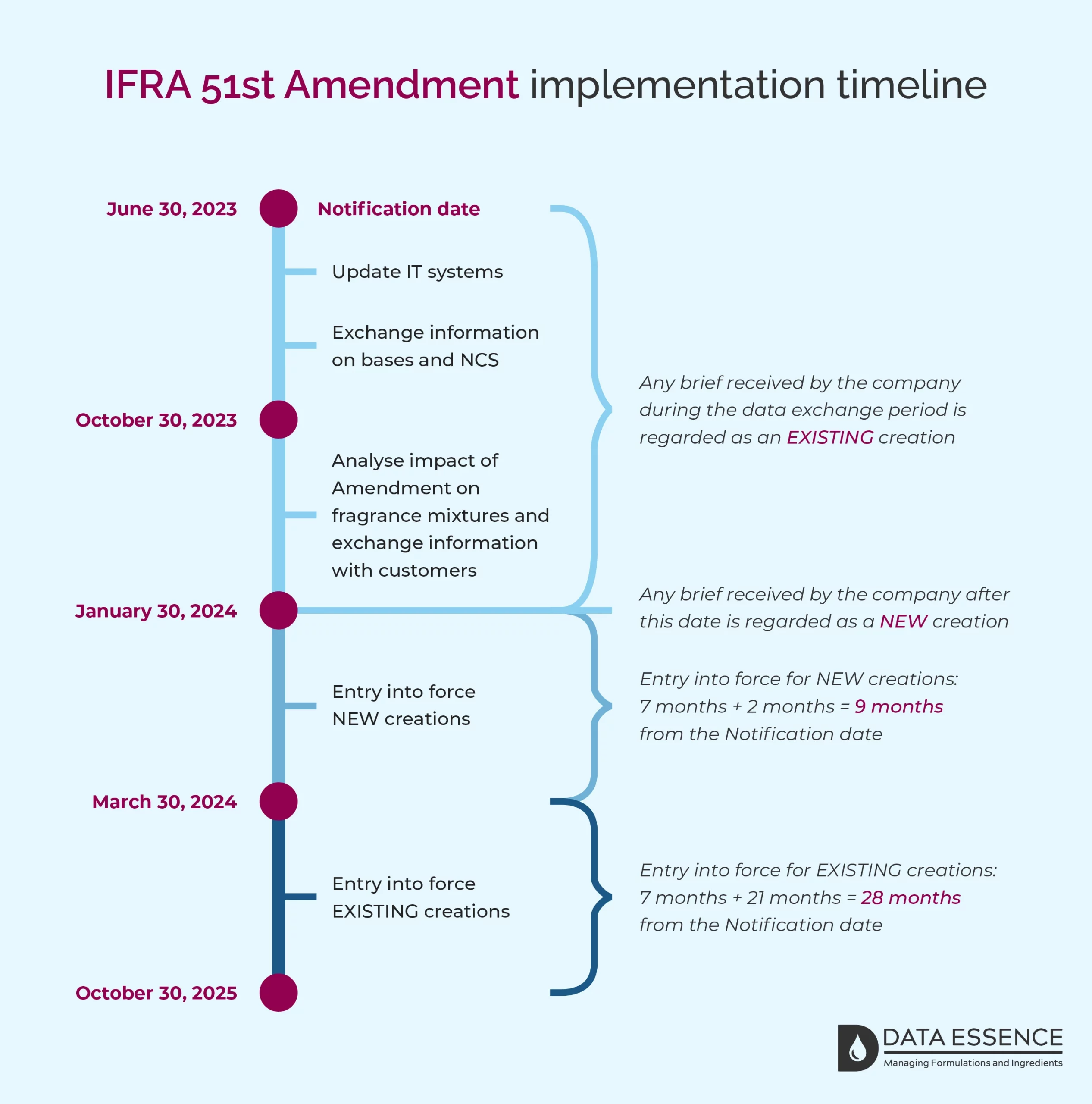
An IFRA Certificate of Conformity in compliance with the 51st Amendment to the IFRA Standards is required by March 30, 2024, for NEW creations and by October 30, 2025, for EXISTING creations. This applies to the new Restriction and Restriction Specification Standards only.
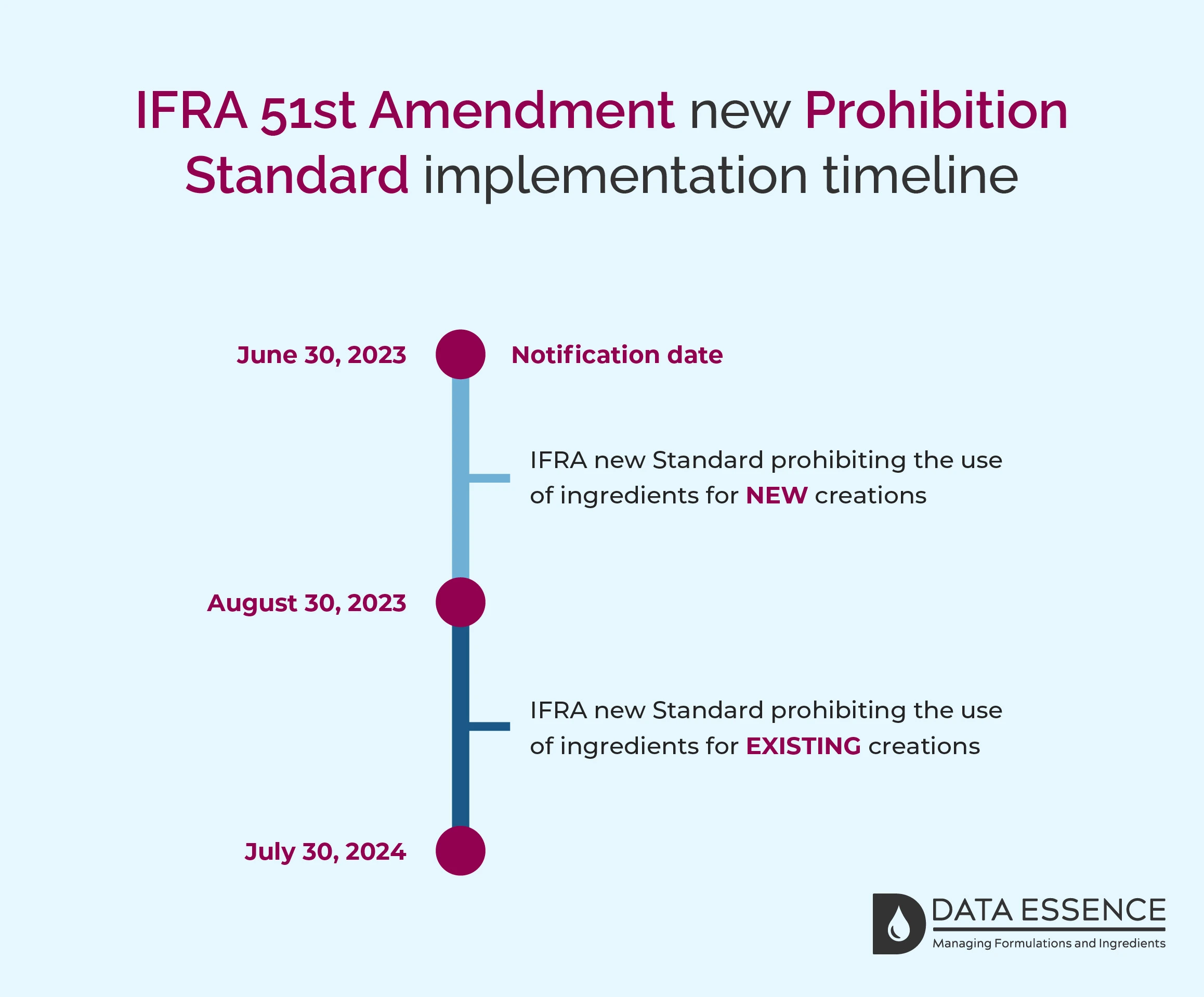
The Amendment includes 1 new Prohibition Standard and the dates for compliance are different. For NEW creations by August 30, 2023, and EXISTING creations by July 30, 2024. These dates apply to the supply of fragrance mixtures (formulas) only, not to the finished consumer products in the marketplace.
What is new with the 51st Amendment?
The Standards used to have, where applicable, a reference to Annex I concerning the presence of the restricted ingredient in Natural Complex Substances (NCS) and to Annex II concerning contribution from Schiff bases. This information is now combined into one single Annex, named ‘Contributions from other sources’ and will only refer to this Annex. These changes are not only introduced to the format of all new and revised Standards that are part of the 51st Amendment but will also be applied to all existing Standards available on the IFRA website, as part of the 51st Amendment.
A summary of relevant changes to the guidance for the use of the IFRA Standards
- Clarification about paper products – clearer wording on how to apply the IFRA Standards for paper products when calculating fragrance ingredient concentrations in finished products.
- Clarification about categorization of fabric softener sheets, dryer sheets and dry-cleaning kits – dryer sheets are now in Category 12. IFRA concluded that fabric softener sheets and dryer sheets are the same and should be mentioned in the same entry in Category 12. Dry-cleaning kits have been classified into 2 types; one that is placed in the dryer and therefore has limited skin contact and could qualify as Category 12 as well, and one that would involve manual application and because of certain skin contact would be best placed in Category 10A.
- Categorization of pillow spray – the placing of pillow spray in category 11B is based on skin sensitization concerns and potential transfer of product from the pillow to the skin.
- Categorization of reed diffusers – some impactful changes to the MAC levels for Category 10A of two revised Standards have a potential impact on reed diffusers.
- Handling of traces – the RMTF felt that the current text is adequate regarding describing the roles and responsibilities of exchanging information along the supply chain as the basis for responsible management of traces in finished consumer products.
- Phototoxicity considerations for products in Category 6 – the Expert Panel for Fragrance Safety concluded that the products in Category 6 can be considered rinse-off with regard to phototoxicity.
- Elimination of ”sunblock” terminology in the guidance – the term ”sunblock” is replaced with the term “UV filters” instead.
- Clarification that make-up remover for face and eyes also includes potential exposure to lips – these products are not considered to have exposure conditions comparable to products designed for lips that must follow the requirements for Category 1. A make-up remover also suitable for lips is still considered similar to a make-up remover marketed for ‘face’ and therefore does not always fall under the requirements as products marketed for leave-on use on lips.
- Clarification about ”aftershaves of all types” in Category 4 – the new descriptor in the guidance document is ”aftershaves of all types (except creams and balms)”. Some aftershaves are cream and lotion products and may be categorized as face moisturizers in Category 5B.
A summary of the relevant changes within the IFRA Categories
- Category 4 – Aftershaves of all types have been amended to exclude creams and balms (which are now assigned to Category 5B).
- Category 5B – beard and moustache care have been added to facial moisturisers and creams.
- Category 8 – now includes intimate deodorant spray.
- Category 10A – includes manually applied dry-cleaning kits.
- Category 11B – includes pillow spray.
- Category 12 – includes dryer/fabric conditioner sheets and dry-cleaning kits added to the dryer (moving out of Category 10A). Toilet gel and scent beads have also been added to this category.
Need help staying compliant with IFRA Standards?
A summary of the new and amended Standards within the 51st Amendment
- Most limits included within the Revised Standards are more restricted than before, with a few exceptions. For example, some limits have increased for Anisaldehyde and 4-Methoxy-alpha-methylbenzenepropanol.
- Given that there are 48 New Standards and 12 Revised Standards, it is essential to review each of these Standards to determine if they may have an implication for any fragrance ingredients and formulations that you create or use in your finished products. The Standards are freely available to members and non-members on the IFRA website.
- Methyl Eugenol and Estragole have lower limits – some NCS will be affected, particularly the use of basil, tarragon, and nutmeg.
- Phototoxicity now applies to rinse off products.
- The restriction standard for p-Cresol refers to ‘mixed cresols’ but does not specifically apply to the isomers o-Cresol and m-Cresol.
- Two new CAS numbers are included for Farnesol (16106-95-9 and 3879-60-5) and one new CAS number for Mintlactone (38049-04-6). Please note that these 2 minor updates to existing Standards are not considered affecting the content of the Standards on Farnesol and Mintlactone. Thus, the implementation timelines associated with the 51st Amendment do not apply for the Standards on Farnesol and Mintlactone.
What remains the same following the IFRA 51st Amendment?
- Over 200 IFRA Standards have not been amended and remain the same.
- Reed Diffusers remain in Category 10A, which some in the industry may differ in opinion with this Categorisation. This may be reviewed in the 52nd Amendment.
Conclusion
The 51st Amendment to the IFRA Standards is an achievement that underscores the fragrance industry’s dedication to safety. By incorporating the latest scientific knowledge, the amendment sets a new standard for fragrance safety while encouraging innovation. While it presents challenges for fragrance manufacturers to reformulate their products and meet stricter safety guidelines, it also opens the door to innovation, environmental responsibility, and improved consumer trust. Consumers can enjoy their favourite scents with peace of mind, knowing that the fragrances they love are created with our well-being and the planet in mind. We recommend visiting the official IFRA website, which provides comprehensive and up-to-date information on the latest standards and amendments.
Need help staying compliant with IFRA Standards?

