What are fragrance allergens?
Recent regulatory changes in the EU Cosmetics Regulation highlight a crucial aspect of these fragrances – the presence of specific substances known as fragrance allergens. These substances can potentially induce contact allergies and sensitisation in susceptible individuals. In response to this health concern, the updated regulation now mandates individual labelling of fragrance allergens if they surpass defined concentration thresholds.
For professionals in the cosmetics industry, comprehending what fragrance allergens are and why their identification on labels is crucial for consumer well-being is imperative. This article explores this essential topic.
What were the labelling requirements for fragrance allergens before the new amendment?
What are the labelling requirements for fragrance allergens with the new amendment?
It is crucial to note that these fragrance allergens must be listed on the ingredients label, regardless of their source. Even though they bear the label ‘fragrance allergens’, these substances must be disclosed on the label, whether they originate from the fragrance component or are intentionally added as specific cosmetic ingredients. This comprehensive approach ensures that consumers have access to vital information about allergenic substances, promoting safety and transparency in the cosmetics and fragrance industry.
When do the amended allergen labelling requirements come into force?
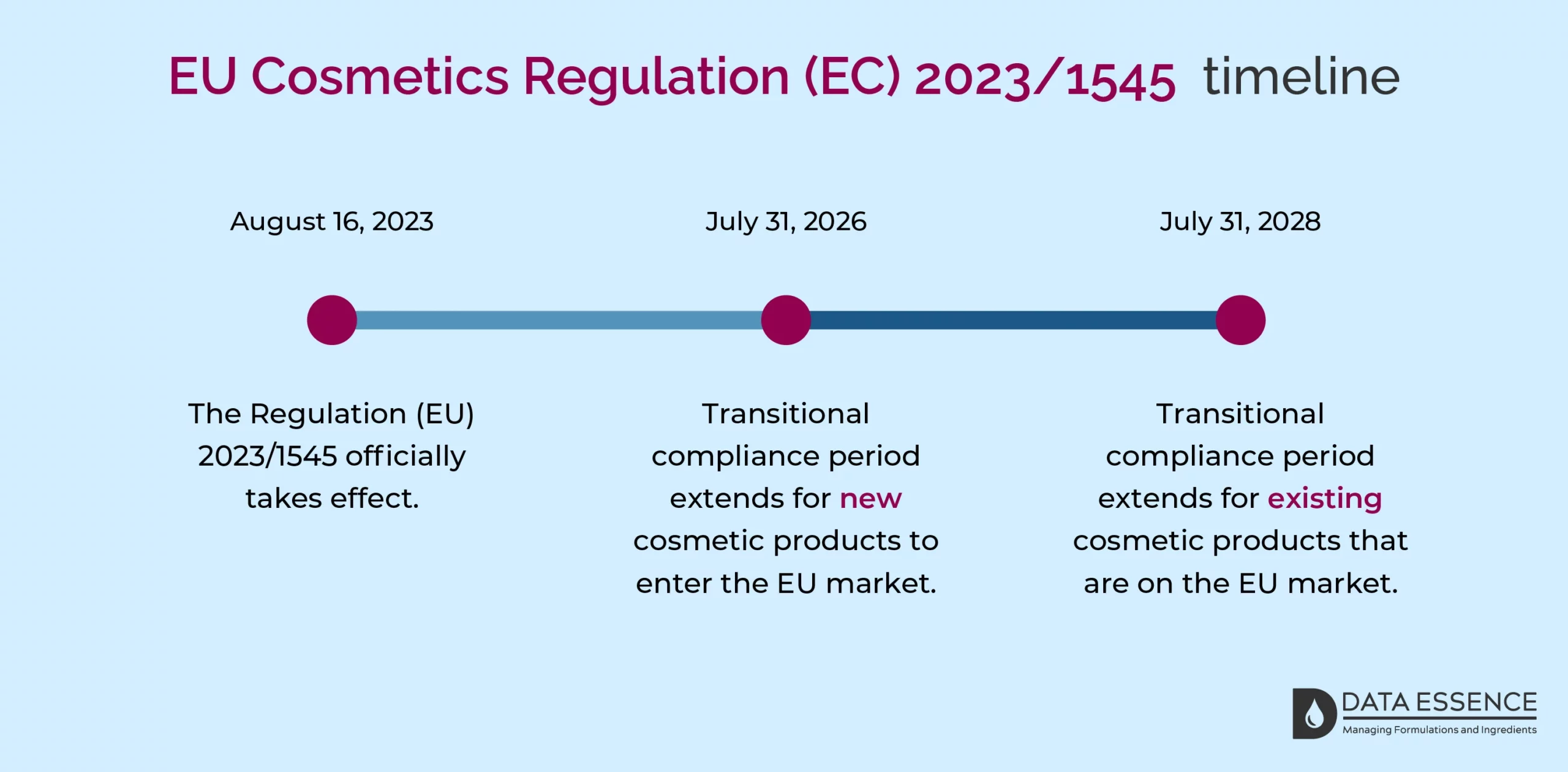
The Regulation (EU) 2023/1545 officially takes effect on August 16, 2023, and applies to all Member States. There is a transitional compliance period to accommodate substantial adjustments, such as revising product formulations, and containers, removing non-compliant items, and issuing new labels.
Have a look at the image below to see how this transition extends for new and existing products.
Where should fragrance allergens be listed on the label?
A common naming system called the International Nomenclature for Cosmetic Ingredients (INCI) is employed to make ingredient identification easy for the public. This ensures consistency in ingredient names across the EU, GB, and most countries worldwide. Ingredients should be listed in descending order of weight when they are added to the cosmetic product. Ingredients in concentrations of less than 1% can be listed in any order after those exceeding 1%. You can list fragrance allergens based on concentration or place them at the end if they are not intentional cosmetic ingredients.
Challenges and adaptations of expanded allergen listings
The expansion of allergen listings has presented fresh challenges in regulations and implementation:
- The challenge of allergic consumers memorising many complex allergen names within the same category.
- Label space constraints due to extensive ingredient lists.
- Implications for globally compatible labelling practices.
To mitigate the complexity of fragrance allergen names and label space constraints, a novel system has been devised. It involves categorising substances with similar allergenic properties under shared group names rather than listing individual substance names. Additionally, to ensure the global effectiveness of these new group names, some substances and the newly introduced group names have received approval for INCI names – International Nomenclature for Cosmetic Ingredients, internationally recognised systematic names for identifying cosmetic ingredients.
Consumer awareness and safety
Consumer advocacy groups and organisations play a vital role in promoting safety by advocating for stricter regulations, raising awareness, and offering guidance to those with allergies. By staying informed and advocating for their rights, consumers contribute to a safer and more transparent cosmetics market, ensuring that their choices align with their sensitivities and preferences.
Conclusion
While these changes may initially pose challenges for cosmetic manufacturers, innovative labelling solutions and a commitment to consumer safety ensure that a diverse range of products remains accessible.

